PLC & CSV Validation
General validation activities with paper and manual-based validation processes cause compliance risk bringing products to the market on time. The validation activities have become important around documentation for ensuring quality. Current validation process requires a high degree of manual effort for tracking and reporting and the execution of most validation protocols takes place on paper and adds value to organization by adopting software that increases productivity and quality.
Pharma SoftSol provides a variety Computer System Validation of services to help your company ensure compliance (FDA, EMEA, MHLW,GAMP etc……..) and offers validation support and documentation.
Validation is applied to systems satisfying regulatory requirements in the Pharmaceutical, healthcare and medical devices industry
CSV includes validation of both new and existing computer systems
A “computer system” is defined as “any programmable device procedures including its software, hardware, peripherals , users, interconnections and inputs for the electronic processing and output of information used for reporting or control.
The validation effort required for computer systems varies based on the -
Level of risk that use of the system presents (direct vs. indirect impact on patients)
Type of system (configurable vs. bespoke)
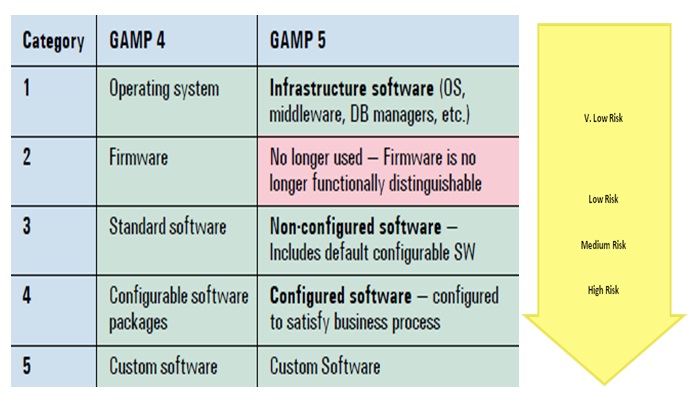
Validation effort and activities are undertaken based on the appropriate GAMP software category.
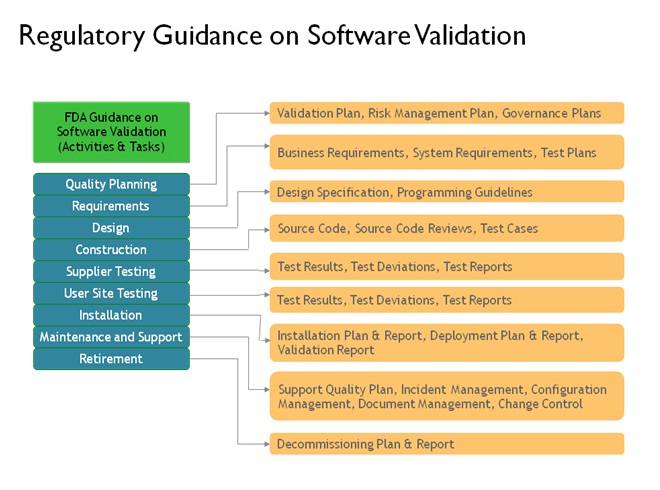
For software used required the following document types or their equivalent information:
Validation Plans and Protocols
Documented Risk Assessment (e.g. critical operating parameters assessment by mentioning Regulatory, Technical and Business impact)
Documented Requirements, Specifications and Designs
Verification activities such as Testing or Qualification according to approved plans or protocols (e.g.; IQ, OQ, PQ)
Control mechanisms to ensure integrity and completeness during the validation or qualification process
Procedures and/or other mechanisms to maintain the system in a controlled state after initial implementation
Reports that provide summaries and conclusions of validation/qualification based on the completion of planned activities
Post project control of the documentation evidencing the completion of validation and/or qualification plans
Companies struggle to create goals, processes, culture, management, and incentives that build infrastructure. Computer Systems (software) are a dominant and increasing part of business infrastructure. Computer System Validation is process validation and has the following benefits:
Excellent Return On Investment (ROI) - Cost of compliance is low with respect to potential loss of intellectual property or other potential losses.
Provides documentation required by FDA,EMEA, other regulatory agencies, and your customers.
Maximizes the value of the computer system and the employees that use it.
Reduces labor costs by increasing employee efficiency and effectiveness.
Enhances project management to ensure projects are implemented on schedule and on budget.
Saves money by discovering defects early, before failures occur in production.
Reduces risk. Legal liability, not regulatory, is often the most important reason to perform validation. Software is made and used by people so it is guaranteed to be imperfect. Software and process defects increase with software complexity. Having the evidence that computer systems are correct for their purpose and operating properly represents a good business practice.
Promotes continual process improvement. Software is constantly evolving to keep up with the increasingly complex needs of the people that use it. Therefore, validation is an ongoing necessity.



